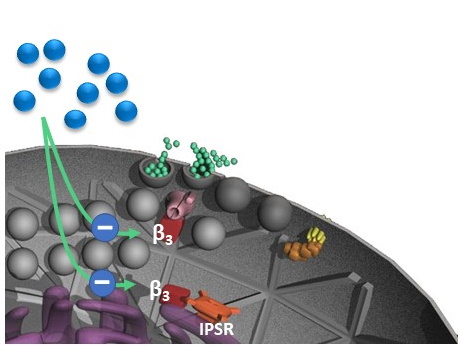
Calcium channels
.As
As glucose is taken up by beta-cells, a chain of molcular events leads to activation of calcium
channels (pink barrels) allowing more calcium ions to flow into the cell. This in turn stimulates
insulin release (green circles) to the blood. In diabetic patients, ß3-subunit (red block) of calcium
channel restrains insulin secretion. To reverse this action, ß3-subunit could be blocked by a
specifically designed substande (blue circles).
Calcium channel drives unique functions of a cell
An ion channel is a complex of proteins embedded in the cell plasma membrane. Each channel allows only specific ions to flow through, so potassium ions are transported through the potassium channel and calcium ions through the calcium channel. The latter is built up of four subunits: the main open-barrel shaped α1 that allows the ions to flow through the plasma membrane and three assisting subunits – α2δ, β and γ. Calcium channel activity drives unique functions in different cell types, such as muscle contraction by muscle cells and insulin secretion in pancreatic beta-cells.
Calcium channel mediates insulin releasing function in pancreatic beta-cells
When levels of blood sugar, or glucose, rise, it is efficiently taken up by beta-cells. Within the cells, glucose initiates a chain of molecular events that lead to calcium channel opening, allowing more calcium ions to flow into the beta-cells. Calcium ions stimulate insulin release to the blood. Thus the higher the blood sugar concentration, the more insulin is released by beta-cells; an effect mediated by Ca2+.
Selective inhibition of beta3-subunit in beta-cells increases insulin release
There are several types of calcium channel β-subunits. One of them, β3, has an unexpected function: it restrains insulin secretion to the blood. By selectively blocking the β3-subunit in beta-cells of diabetic patients, one should increase insulin secretion promoted by high levels of glucose, and prolong beta-cell survival.
.
.
.
.
.
.
Read more about calcium channels:
- The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology. Yang SN and Berggren PO. Endocrine Reviews 2006 Oct;27(6):621–676
- Beta-cell CaV channel regulation in physiology and pathophysiology. Yang SN and Berggren PO. Am J Physiol Endocrinol Metab. 2005 Jan;288(1):E16-28
- Removal of Ca2+ channel beta3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Berggren PO, et al. Cell.2004 Oct 15;119(2):273-84
- Enhanced expression of β cell Ca 3.1 channels impairs insulin release and glucose homeostasis. Yu J et al. Proc Natl Acad Sci U S A. 2020 Jan 7;117(1):448-453.
- IgGs from patients with amyotrophic lateral sclerosis and diabetes target Ca α δ1 subunits impairing islet cell function and survival. Shi Y et al. Proc Natl Acad Sci U S A. 2019 Dec 11;116(52):26816-22.
- The eye as a novel imaging site in diabetes research. Yang SN and Berggren PO. Pharmacol Ther. 2019 May;197:103-121.
- Blocking Ca Channel β Subunit Reverses Diabetes. Lee K et al. Cell Rep. 2018 Jul 24;24(4):922-934.
- TLR3-/4-Priming Differentially Promotes Ca(2+) Signaling and Cytokine Expression and Ca(2+)-Dependently AugmentsCytokine Release in hMSCs. Park KS et al. Sci Rep. 2016 Mar 16;6:23103.
- CaV1.2 and CaV1.3 channel hyperactivation in mouse islet β cells exposed to type 1 diabetic serum. Yang G et al. Cell Mol Life Sci. 2015 Mar;72(6):1197-207.
- Ionic mechanisms in pancreatic β-cell signaling. Yang SN et al. Cell Mol Life Sci. 2014 Nov;71(21):4149-77.
- Apolipoprotein CIII hyperactivates β cell CaV1 channels through SR-BI/β1 integrin-dependent coactivation of PKA and Src. Shi Y et al. Cell Mol Life Sci. 2014 Apr;71(7):1289-303.
- Glutamate is a positive autocrine signal for glucagon release. Cabrera O et al. Cell Metab. 2008 Jun;7(6):545-54.
- Requirement of inositol pyrophosphates for full exocytotic capacity in pancreatic beta cells. Illies C et al. Science. 2007 Nov 23;318(5854):1299-302.
- Glucose recruits K(ATP) channels via non-insulin-containing dense-core granules. Yang SN et al. Metab. 2007 Sep;6(3):217-28.
- Transthyretin constitutes a functional component in pancreatic beta-cell stimulus-secretion coupling. Refai E et al. Proc Natl Acad Sci U S A. 2005 Nov 22;102(47):17020-5.
- CaV2.3 channel and PKClambda: new players in insulin secretion. Yang SN and Berggren PO. J Clin Invest. 2005 Jan;115(1):16-20.
- Apolipoprotein CIII promotes Ca2+-dependent beta cell death in type 1 diabetes. Juntti-Berggren L et al. Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10090-4.
- Growth hormone promotes Ca(2+)-induced Ca2+ release in insulin-secreting cells by ryanodine receptor tyrosinephosphorylation. Zhang Q et al. Mol Endocrinol. 2004 Jul;18(7):1658-69.
- Cytosolic multiple inositol polyphosphate phosphatase in the regulation of cytoplasmic free Ca2+ concentration. Yu J et al. J Biol Chem. 2003 Nov 21;278(47):46210-8.
- Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic betacells. Olsen HL et al. J. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5187-92.
- Modulation of L-type Ca(2+) channels by distinct domains within SNAP-25. Ji J et al. Diabetes. 2002 May;51(5):1425-36.
- Inositol hexakisphosphate increases L-type Ca2+ channel activity by stimulation of adenylyl cyclase. Yang SN et al. FASEB J. 2001 Aug;15(10):1753-63.
- Effects of serum from patients with type 1 diabetes on primary cerebellar granule cells. Chandra J et al. Diabetes. 2001 Feb;50 Suppl 1:S77-81.
- Imidazoline compounds protect against interleukin 1beta-induced beta-cell apoptosis. Zaitsev SV et al. Diabetes. 2001 Feb;50 Suppl 1:S70-6.
- Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic beta -cell. Zhang W et al. J Biol Chem. 2000 Dec 29;275(52):41521-7.
- Synaptotagmin III isoform is compartmentalized in pancreatic beta-cells and has a functional role in exocytosis. Brown H et al. Diabetes. 2000 Mar;49(3):383-91.
- Syntaxin 1 interacts with the L(D) subtype of voltage-gated Ca(2+) channels in pancreatic beta cells. Yang SN et al. Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):10164-9.
- Cysteine string protein (CSP) is an insulin secretory granule-associated protein regulating beta-cell exocytosis. Brown H et al. EMBO J. 1998 Sep 1;17(17):5048-58.